Three main research branches
My research group at the Medical University of Vienne (MedUniWien) focuses on three main topics:
- BrainBuild: Human fetal brain malformations
- BrainPRC2: The role of Polycomb in brain development
- EnvironMatters: mechanisms of pediatric brain tumor development in the context of tumor predisposition syndromes
My current funding:




Mechanisms of human fetal brain development
The characterization of distinct cell types in human fetal brain malformations is surprisingly superficial. In order to overcome this knowledge gap, I am making use of my knowledge on particular cell type markers to visualize the various progenitor, neuron, and glia populations in fetal tissue archived in the impressive biobank at the Division of Neuropathology and Neurochemistry at MedUniWien.
The resulting detailed description of cellular alterations in distinct malformations, such as hemimegalencephaly, will be highly valuable to the community as important reference for experimental models besides awareness about our biobank for research purposes.
Happy to collaborate!
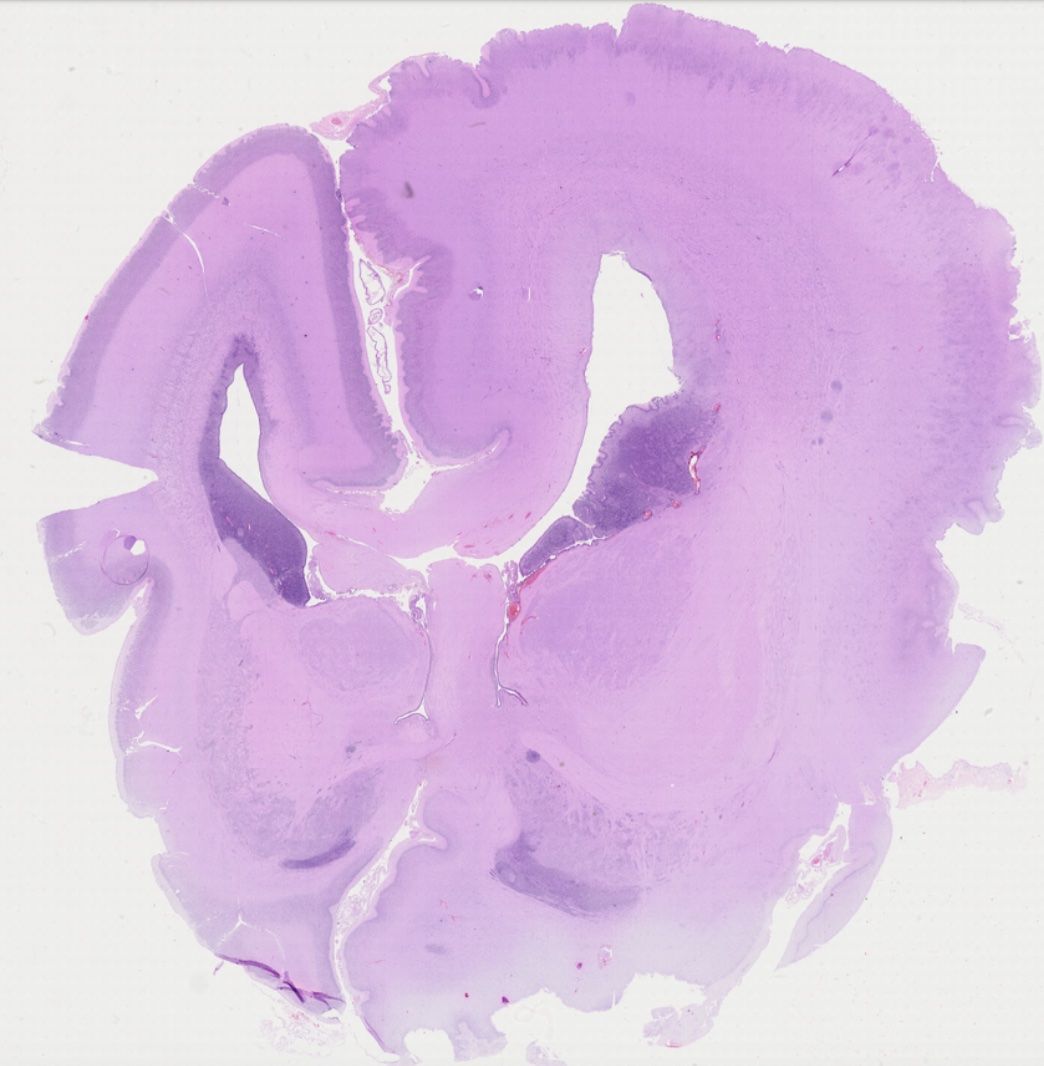
GW22 hemimegalencephaly
Mechanisms of pediatric brain tumor development
This part of my research focuses on dissecting the cell-autonomous and non-cell-autonomous mechanisms of pediatric brain tumor development in patients suffering from tumor predisposition syndromes. These syndromes are caused by germline mutations and account for 8-19% of pediatric brain tumors, of which some show highly aggressive behavior and are thus associated with a poor prognosis. In contrast to spontaneous somatic mutations, germline mutations provide a particularly challenging setting for the precise assessment of tumor-driving mechanisms based on an all-mutant cellular context of the organism.
A rigorous dissection of potentially multiple entangled cell-autonomous and global-tissue mechanisms downstream of the mutations is thus required to precisely assess the contribution of the cellular environment to tumor formation across developmental time. I hypothesize (1) that a non-mutant stem cell niche can provide fundamental control mechanisms preventing or slowing down tumor development and (2) that the identification of these control mechanisms can be used to design novel targeted therapies for syndromic patients.
Being positioned in the clinical setting of the Division of Neuropathology and Neurochemistry at the Medical University of Vienna grants me direct access to precious patient material and provides me with excellent state-of-the-art expertise in neuropathology and molecular diagnostics.
I aim to systematically and functionally address these hypotheses through a combination of molecular analysis of patient biopsies and the establishment of experimental platforms. These platforms include the generation patient-derived organoids and complimentary mouse models using the elegant Mosaic Analysis with Double Markers (MADM) technology established by Hui Zong and further expanded by Simon Hippenmeyer) allowing to assess stem cell behavior at single cell resolution.
Altogether, my research aims to answer the following open questions:
- What are the quantitative principles during morphogenesis of distinct brain regions from an individual stem cell’s perspective?
- What is the precise identity/state of a mutant neural stem cell when undergoing oncogenic transformation?
- What is the sensitive developmental time window for oncogenic transformation?
- How are the above mentioned parameters (individual stem cell behavior, mutant stem cell state and tumor initiation) shaped by cell-autonomous (i.e. cell intrinsic) and non-cell-autonomous (i.e. systemic or tissue-wide) cues elicited by the mutant cell(s)?
- Can we identify universal tissue-wide laws governing stem cell proliferation and differentiation in distinct brain regions?
- What are the divergent pathways among particular tumor predisposition conferring tumor-specific features such as aggressiveness?
Here is a glimpse into our first patient-derived iPSC lines and cerebral organoids:
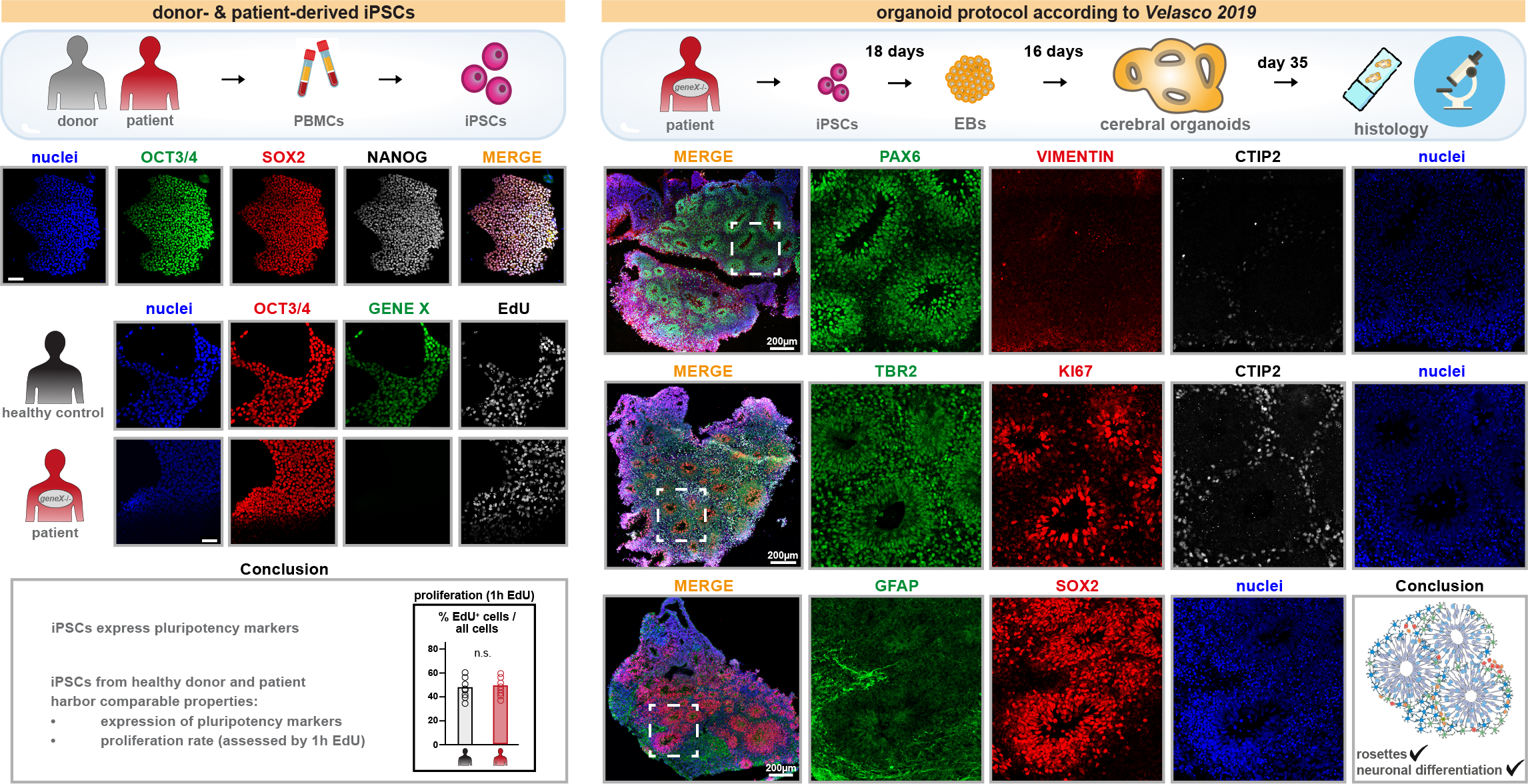
Foundation:
My postdoctoral work uncovering distinct and sequential functions of the epigenetic regulator Polycomb Repressive Complex (PRC)2 in cortical development provides a compelling framework for my hypothesis towards the existence of – potentially region- or cell type-specific – tissue-wide control mechanisms keeping individual mutant stem cells in check when existing in a non-mutant environment.
Briefly, I have discovered that PRC2 is not cell-autonomously required for faithful neurogenesis, but rather orchestrates neuron production on the global tissue level in a non-cell-autonomous manner (see Figure below, top level). In other words, I have shown that global tissue effects mediated by a PRC2-sufficient environment provide protective mechanisms to mutant stem cells and instruct normal neurogenic behavior in individual mutant cells. However, at later stages of cortical development, namely astrogliogenesis, single mutant cells do require cell-autonomous PRC2 function for proper astrocyte progenitor proliferation and astrocyte maturation (see Figure below, bottom panel).
The established experimental assays from my postdoctoral work thus provide the profound stepping stones for evaluating the instructive cues mediated by the cellular environment for other essential genes/cellular processes in brain development.

Distinct and sequential PRC2 functions in cortical stem cell lineage progression. Top panel: PRC2 does not cell-autonomously govern excitatory projection neuron production, but is required on the global tissue level. As a result, sparse PRC2-/- stem cells produce a normal amount of neurons, while tissue-wide loss of PRC2 results in reduced neuron production and microcephaly. Bottom panel: PRC2 cell-autonomously controls cortical astrocyte production and astrocyte maturation. Individual PRC2-/- astrocyte progenitors undergo less rounds of proliferation. Furthermore, PRC2-/- astrocytes show reduced morphological complexity.